Dr. Thomas Pulinilkunnil
PhD (UBC, Canada) ProfessorDepartment of Biochemistry and Molecular Biology, Dalhousie University
Department member since 2012
Postdoctoral fellowship – University of Alberta, AB, Canada, 2008-2012
Postdoctoral fellowship – Harvard Medical School, MA, USA, 2005-2008
PhD – University of British Columbia, BC, Canada, 2005
M.S. (Pharm) – NIPER, Mohali, India, 1999

Research Areas
Proteotoxic Basis for Metabolic Heart Disease
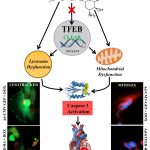
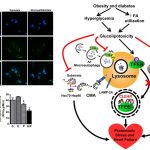
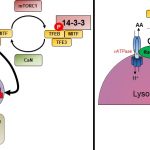
One of the major focus of the Pulinilkunnil laboratory is to assess how adaptive or causative changes in protein synthesis and degradation causes heart disease. To maintain cellular homeostasis, synthesized proteins are degraded and recycled inside cellular organelles called lysosomes by a process known as autophagy. Using a multispecies approach (rodents, yeast and zebrafish) Pulinilkunnil lab will specifically examine how different drugs, hormones and nutrients (glucose, fat and amino acids) influence lysosomal function to alter autophagy. Furthermore investigate whether aberrant lysosomal function impact mitochondrial metabolism and energetics thus governing cardiovascular outcomes in biology and disease.
Lysosome Nutrient Sensing and Metabolism in Cancer Pathogenesis
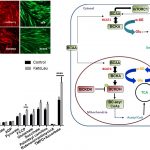
Reprogrammed energetics and metabolism is an emerging hallmark of cancer cells. Indeed, altered mitochondrial fuel metabolism contributes to oncogenic mutations which in turn influences cellular signaling and function. Numerous oncogenic processes that are resistant to chemotherapeutics are found to exhibit features of intermittent oxygen and nutrient deprivation, mitochondrial stress, abnormal cell growth and suppressed cellular death. Recent studies have shown that abnormalities in cellular metabolism augment autophagy to facilitate cancer cells to precipitously adapt to environmental stressors by sustaining uninterrupted proliferation thereby evading demise by radiation and/or chemotherapy. Our laboratory is currently elucidating the mechanism by which tumor cell metabolism signals changes in lysosomal autophagy and mechanisms by which this signaling could influence the outcomes of treating cancer. By specifically examining the cross talk between mitochondria and lysosome we hope to uncover novel biochemical pathways that could be targeted selectively to render cancer cells susceptible to first line cancer treatment.
Branch Chain Amino Acid Regulation of Energy Metabolism
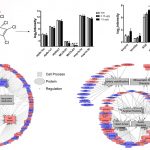
Amino acids particularly branched-chain amino acids (BCAA) such as leucine, isoleucine, and valine are mostly used for manufacturing proteins for growth within muscle but are also used for generating energy. However, our understanding of how BCAA are utilized for generating energy within cells is still lacking. Goals of our research are to determine the enzymes mediating this process, how these enzymes are regulated and the impact on the cell when BCAA are used as an energy source. We want to know whether BCAA compete with other nutrients for being consumed by the cell for energy and which pathways regulate the competition process. Despite the widespread presence of amino acid metabolizing enzymes in different tissues their impact on mitochondrial energy generation and function in health and disease remains unexplored. Using cell biology, genetic and whole body approaches in zebrafish and mouse my research program will focus broadly on addressing the following questions; 1) to uncover how the nutrient triad glucose, fatty acid and BCAA biochemically communicate with each other resulting in nutrient transport and utilization within the mitochondria; 2) to decipher whether within the cell different biomolecules such as ions, hormones, lipids, sugars and neurotransmitters employ the services of amino acids to govern cellular growth and survival in health and disease.
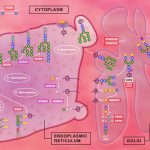
Biology of ER Glycosylation in Health and Disease
Glycosylation is an extremely important function by which all human cells build sugar chains or glycans that are subsequently attached to other functional molecules, including proteins and lipids. The products of these attachments are called glycoproteins or glycolipids, and are required for the normal growth and function of all tissues and organs. Impairment in glycosylating enzyme disrupts glycan synthesis and metabolism leading to congenital disorder of glycosylation (CDG). Using yeast and zebrafish models Pulinilkunnil lab aims to examine molecular pathways by which impaired glycosylation promotes intracellular distress specifically in organelles like mitochondria, endoplasmic reticulum and lysosomes that are mainly responsible for generating energy, performing quality check on proteins and degrading cellular waste. This research will identify and characterize novel pathways and proteins mediating pathological effects of defective glycosylation.
Lysosome, Autophagy, Metabolism, Energetics, Amino Acids, BCAA, Obesity, Diabetes, Breast Cancer, Lipids, Glycosylation, Zebrafish, Heart failure